Originally Posted By: RDY4WAR
First post. Been lurking the site for years and finally joined.
I've been researching this for a little while. I know a little about their function, that primary has a slower consumption rate but harder to activate, and secondary is the opposite. What I'm hoping to find out is the difference in molecular structure that distinguishes one from the other. Also, in what applications is each one used?
Is one found in gas oil and other in diesel oil?
Does one brand use one over the other?
Do specialty oils (break-in, racing, etc...) use one over the other?
Is primary even found in engine oil? (doesn't sound like it would be favorable)

Here is the structure of the ZDDP, with the four R's being the alkyl groups. Alkyl groups are simple C - H - C - H chains such as methyl (1 C), ethyl (2 C's), propyl (3 C's), butyl (4 C's), ... ZDDP alkyl chains are characterized by how many carbon atoms are in the chain (it could be anywhere from a few to more than a dozen) and if the carbon atom that attaches to the ZDDP molecule is primary or secondary. Carbon atoms make four bonds. Primary carbon atom means that only one of the bonds is another carbon (the remaining being two hydrogen atoms and the ZDDP molecule); secondary carbon atom means two of the bonds are other carbon atoms (the remaining being one hydrogen and the ZDDP molecule), and tertiary carbon atom means three of the bonds are other carbon atoms (the remaining being the ZDDP molecule). Bonds to primary carbon atoms are more stable than bonds to secondary carbon atoms, which are in turn more stable than bonds to tertiary carbons atoms, as hydrogen bonds are more difficult (require more energy) to break than carbon bonds.

There are also ZDDP's that use aryl groups (aromatic carbon rings) instead of alkyl groups (carbon chains).
These are some additional compounds used instead of ZDDP:
ZDDP's are classified according to the number of carbon atoms in the alkyl chain and whether the bonding carbon is primary, secondary, or tertiary. You can have a mix as well, as there are four alkyl chains and some can be primary while others can be secondary. There can also be a different number of carbon atoms in each of the four alkyl chains.
Regarding the antiwear ability, there is no clear answer. Some say secondary ZDDP is better. However, this paper found that primary ZDDP with 8-carbon alkyl chains (P8 ZDDP) was best when used in combination with dinuclear moly DTC. So, there is no clear answer in regard to which ZDDP is better in terms of antiwear ability. It's complicated.
(
Link) Influence of the alkyl group of zinc dialkyldithiophosphate on the frictional characteristics of molybdenum dialkyldithiocarbamate under sliding conditions
Masayoshi Murakia ^a and Hisayuki Wadab ^b
^a Department of Mechanical Engineering, Shonan Institute of Technology, 1-1-25 Tsujido Nishikaigan, Fujisawa, Kanagawa 251-8511, Japan
^b Lubricants Research Laboratory, Nippon Mitsubishi Oil Corporation, 8 Chidori-cho, Naka-ku, Yokohama 231-0815, Japan
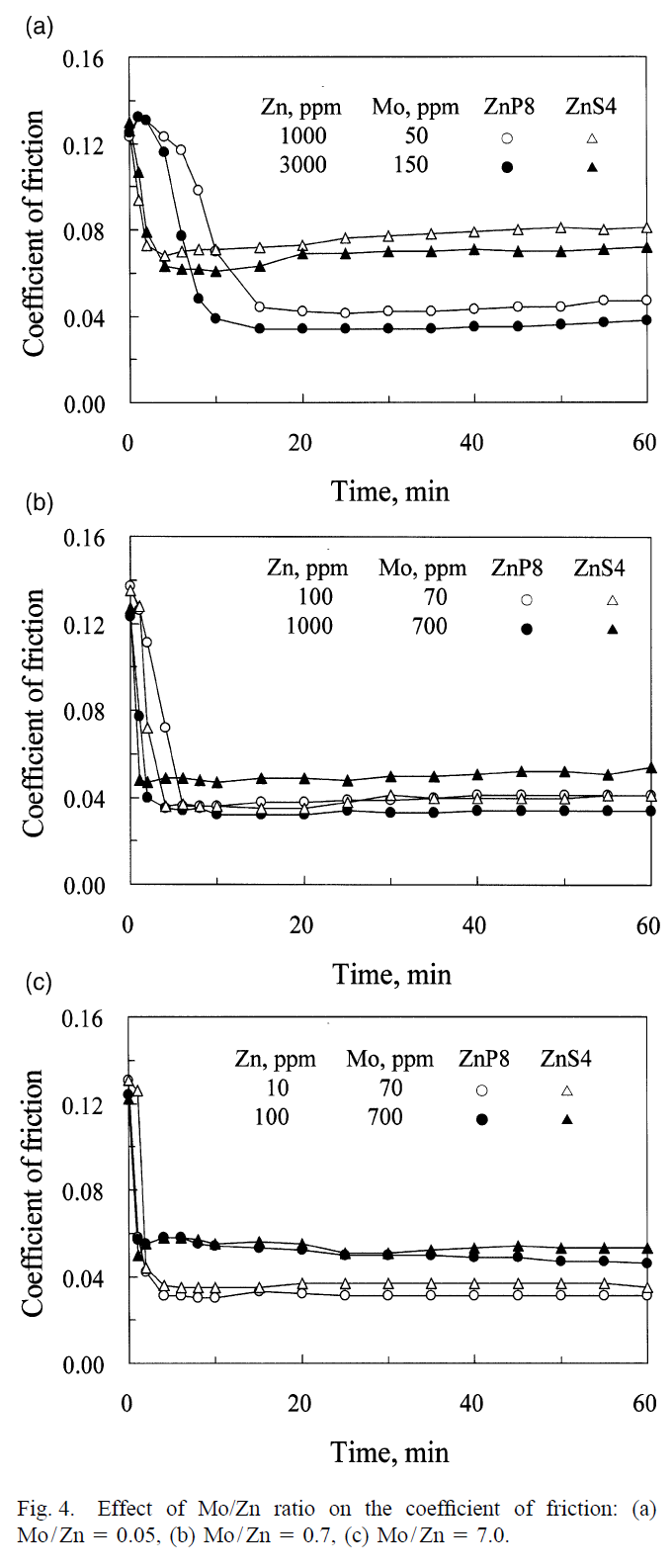
The article studies three different types of ZDDP (two primary and one secondary) along with dinuclear moly for various moly concentrations. The optimal Mo concentration could be as little as 200 ppm or as high as 700 ppm, depending on the type of ZDDP. Also, different ZDDP types lead to different minimum friction coefficient (and antiwear properties, which may get better or worse as friction gets lowered). They found that primary-8-carbon-alkyl-chain ZDDP was best in terms of friction reduction because its better temperature stability and slower decomposition and activation resulted in more molybdenum atoms being embedded in the antiwear film while this film formed, increasing the effectiveness of moly. So, it's not necessarily true that a faster-decomposing secondary ZDDP provides better antiwear. When the synergistic effect of other antiwear/extreme pressure/friction modifier (AW/EP/FM) additives are considered, primary ZDDP could be better, as it competes less with other additives due to its slower decomposition and activation resulting from its better temperature stability.








![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/dWNbOuLwN_ZwY2V_1htvt0mpskN8UMfFCObv825hILHPyEDzoJoBIrODIDHG5__CPGIzBrc3ksAk_Y8IOE__PNczJbFLVSEtQGA94YUjcCX2fQ521cDnqU8dXSxrlMugkef0rLn2wYCz38F2Ee2fZoZKphgiMvN2TAtSqeqEZsnuM87ehM0AYjcJcuKMS-BlYx7nwNiePiVYjvwJptrlNr32P1ZZPxvhGdsH9l_aaiLfO-FKXzXkVeC8mO_0EfUWAeytJI1liEs_5yaPl5qEdayKQ-2YNzTttm91NRckyN5F6GGkdmCpUNVu6YwdKXPIukpW5fIoSa3qlC1aYDalAbF8UI3BjV0qJAeVqrvTHde1OF4jYtdZB6u0aDMWHtZb1ILL0kQim-VfkESSSR4rFaYSWQERYtRUdfzMC9rax24E4Hc6vNFrmjkVuMtc3Mw2U2vTuYIeM6YgfTvU2zGmcpqT0Vm1yE9cdHJIXdSrYe_jL4pkCVoJGCTO6mt9gYZH3Gw6s-BB4Fl6hIswUsWe3b7Yy49iFCQqjo-mEw2CbLMZAoVW1hjftaZAlk7O--nwPMR3JGHxHizHGWGDdn3uwou_Oq3FUblQrHcSqnbdgmadboF7PBkFoyxuhtrB7Qjb=w693-h755-no?.png)
![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/ZeLza6TtfW7uhlGtvsLyg7G5jfkJhO_2gRAknjDVytt_M_nGs8ujVXYygwGMG6G4XQ_UlmohITXm4twD2R3OpD68ICEaiS9KZubXngt-hdWnVADTuq0fkS4vhGUIDoJbnHqxOJhtJgsDIgEteCCw8on_TGyGlYOaU245yIGCoCCxQ0xRq3b82QNEpkXcgmV4d9ZG-QCzYTLMC6nSZgdsy3aLXRq9RSIC2gabe-t5oIEZUWmb0WFecERkKTOBLaO0n8unqkYgrHVT_c8J39rus5WV9by8j4zQbzhHt15SexvaHphlh_MTCFYhP9hbGW93Buu5ICkyiWzP0FyYNCK8j9TtW2m3hgMwe_g9sRVNuscpiT7HCsnu_Q-A6L98vHcuEehrK_ms9PnDmTGzGa3igz-zhgyPBfbAoPnAXuBx0kt91QEErOdXAwLj6VSPPVuAmNDoMZbI8AA34BDyIbNQxGF2CRSkd0czaXERVFJ8LV0WG3k67UDzWEUihNtxDR3tNs0mCtXJxW_VAXsy4DznzzKTwkeApFaL4CTLfnrTNlGbNa4EZfoQFYj3WhXVvRoUQCoVzu01CBeEEdQNX8VhxUZssHbjqBo2e_Pwu3M7JEjNszYrV-vVuNKWKyWuqInr=w724-h876-no?.png)
![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/2T26hUVRP94gTP1g4M6weJS1mbtAsa4s7WmWQKWz3r6lUwU05mlCztAAAXIz3UQ7gj54Kycn1eL6Lu8sVfmlVAZzVJfJQ-_ABbl0-1srn6plFoNwdtvLLnrPO18HasAo6I0zIPil91bIR3bXZnShJ3W4DgrZ38qbQ83BSPNxw1_ueM6AXc7JZzwjZ0o9Anssbd1LBFFkW3XPqds4eBjhH42TkTdRxufNA9W2JHauc4PmF4IG6Bt0lAz3haJOhg8EX8_sB4PKzHxrevLFFu3_gXQWdVdOJrZVZ5BOWwMeOfrmq8UHS9ZClreX0Fe1_nD_mUF1fzTEOzH3TUHolFqv88YMOmkZJdtoZ-qZJ5PryuBoFejWKvyBi8L6qiCJJfkCoxb9jxacXBNxikDgg5-ZpscJXYMhb_0AWPNKz6g7hPi_mJo6CdQsmCD8mioJATC1h3Xq7uyhX0QOtctUSprBZX9y0FfyqY8u05vzE_3S4LdVvXxIjBx3YwZ8ZtEKO3NalL9hlGyAbVrBNgO19InvL6G9gnX4lWBG7zTbiFcql0crPXViofQptY0l3tYd8KRAoFulyaqDGVLAUPn1hNGtt0PSeyq8b88fby_cJfa7JH_QfMMP829QO5N4tGT27NQC=w709-h528-no?.png)
![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/IikdX04iL35V2pWlLvSqoslAMo3vRjjQYDHDy_B0hWQWFVI4oUin3ecdUAV9lmZrLspDG_0ZeclaziBeBLFRchpatUBuEuByy__E8-q2Mcin9MQxcU_vK2B1gBf7FnJs39Zaa5HuaqwDOkRkUwKQyHRSRy3XF1xxMxhcsXKEtTeqzmXU89dPolb3nFc-QWqXAJ3qiEninuHDB87a-n8SEClkm6njWakaSoYdx0-fqycEcUWwOGBDAv4VCtmS-xbWzebj-YdES2CDm1-3gyrisxYiNvdsVJq8Yp5lb5FcQ5EPHWnk1IcDpBdN6wHWIrjDQieHQ_m-Rt7kaMNqP-CIXaXbAbuu3YjsDUGG2dEbH4iW7ZZ4CUvqITTOp6ZypgDFOkyUaAJ-BGTcEtMJBqI_RAuBezIQV2sBcfD5SF_g44HeGVnZxvftjmHgUYC9U6jNjZWDwdOa3NZ6grNudjccxeS0kpH_ychgKhqrycGf8Z4XyudCmMMS1CnwVrTiiaS6qoMNdFd3DFqE9XrTl0h4HuxWL7f4DS24fImqKtGPM8pJkt8noO9xnc5XlvnJstnx8OLTzxIQdec0BDaK0-_8IqvmkK6jBmKKrlRYe_x8cBJXUMsKU998k94kKuEvkbaY=w1032-h567-no?.png)
![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/lBs_q2dhWlwV9er83Jnzy2dG0PJE08hh8NNiksD6_ZKj4CmGamVntL5bjVWG4osh5Iy3leB7AuQymky_-kPBJM4rPXKoIKiDfM4Uz2wdSU_BzzhMChcKFSVuzpcLGdrdpxzxBsPmLgPgX1BzTVrhZuTM4bhmVfdXB6HlZJBRLT73krStmHwdSJZ_HIGvgn7rphmHIqYCM3uexy47MzSRZUVOf5MKaDHlnND-HKLqF3gnDnvKodp0K1Le8oGIWhtpoFyoYgo2qGerI2eXAcioB3n6rp_nY49f4DWqs8lIV6rMG6XJP1YO1_vFZmK5IRDBtl3gCv3FfjdWv4ea8H-HLOkMVJEht3SA6rYdZ-7YuJEl7ciprfdubK_977idlU7aJ4cAHalGlPVGPmffcHySIs1s09ov3ZYZlIIVJl4osSQALpXYUD-caW-i4Wf8__TPHfghYJ3AV5fFTc7o20hqoM4dlJlbJsmVDTJg_awEmSyu66AW5xb827jqqDg2pqr1g9y9D1nsS9pgP1bLzw7SblSgxEhhUM4VPNBphfYgdZ6PAcxjkUDUD_Wt3PlvJHGV_nWEm_8XMqVQPzzbqbkN1sPMWPhtev6KuBEwR5yQkjOJpL4LLtpNvgeosYWZTv6c=w1323-h1897-no?.png)
![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/OLqMTOS_M4CWVcUjirYwpIYlpTfpX2-_Y_QY1zc2bn2HzfNb6num7tbDQRPgL_9qpXgXghn5x_cA-n8IRqSBcN0hW11WExcV6zwPhnTyDjs7jpWW27YPRaG0jwD3t72T_MyeUid2lfp2RhQ1TpnK29IeoQGDSnY5l0_81U7cWFDOO5hiz1P2mQ629pIqfwlvjfCuX4IxfO-CexyKDNgDW2I4zf66eEdyRVUTYhnU8qZACRZf5Bx2flUK1eX8QFHVy4nP1Lc0fSd1UlFEKUxV_fGWf9wVnO4aaijXnb9AjEFN1J7qkF0ufhXd7PVz7p9R7UZ3OY3xh6RPaS864_ae21foW4x2JaVv9QcuSfjBpmeXbPEsI1uj0t8iqq38Y4lDkGZ3qM_56tIjZ7tME4n_LFmekAqyZfeE5Aoi2mowV7LKc8Et9eKkNI0IdYjfW8WaUVVN7MomLpygVVp217qt9gGexJjvqHnbSKEXypH-sl6W8dPGSnJjg3_K-CzKU0cXGFKjef50-heqmQP3YVBFNYlV32zOUXJJn280yx7vx8s7DEQ5tvl6xZ0C0DgrZVefJWmO346GJkBfgHYlnzmWLKigJGZjtaC0oAx3rEXkgiFXoNtCjdwrEBTAtTbdoQZc=w1446-h661-no?.png)
![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/freBKpLDUUPDWsofJ9P0BvTroSC1zV2QJl52KRLkiMyCPD17nbnG1pr3UMbf8WOCnYEL_ZWqOpbIpSJVDiIIFLhQHcXX5yS8XBJbWyLJ5bKa7IDyUh38JVKBNOIaftJJ0xwTyHWz3OyR1FbQXOBIXeTR40NFkZaoQCleaHMvnm2IX2WHEgxibgkw383FXMxjZWvJoF3C8q78UeNOTExJFYuOthu1LZPbN2iXTbuN7aimnRqqJsKQ6j-lz3NRRjbS7gUz5ZyQpWzXHC2l1T8aFjyWiS6T7OFleNKCvrAULKdLGjsfI8MG98M8vQchHHDRyy6CZ2RenekrbrbUUPiopFhvjk0qrVPZh03iXgAZma2bwN5_h8ODuc7Smk9EYdzAgbi1rNeH_KTJCY9EZyOpkMCLVJe2BNUwX4FNwEusWfg_J4Oyi2ORwwcc-br97Kn_FAItUisZnhRQT4X383csWN8xz3O2TSEAB7Zr7vaJp0rejZxFJCmMagEBjDPbzHVmB1BlUwaXYSjTKvhlZ7uvkxpMgFd6iOyZHaDPphVcGlJz_5JiVJuX98E2wdoWQQOVQLIAi-zb4B16LF8d0uW473OToRUq203-rCIuf0qvM4KPd5QQiHwmbB9KdONbip-R=w1440-h1989-no?.png)
![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/EE4zVqXEXPxa1f8_c20mfgB9yHej4jTQtWHLAsROM8gEmbsvRjqH9w8RdNsgJ-MBr_Szez4-L42akd_GBEIpR-aOoeTVLMDggXDpTRVan9MDW3F0exHjPKrrd0TDQnMZp5-LVi7TinrsXtNf1yfrzkVkw936p8m78ruHyxhmjlWoWfLI4PRxzdK-Z-H3-G-wYT2NcVVFNGoNbMIHD47jTc9MwLWk-PZvCRH4IdzNCcPxN21LWfP0YcyHCliFbgd4r2KE_cQMPE9hQhe_-F5ams35XJTfUmysJWnYH6Kljzqvqv8mNNbowb4uJniWKKfKLj9LQwbu6e8ic6DylanTsCWgDb55bdJdmpjpG90iHtJtCfxLTy9xj0_lPsALfOAC9eRnweiwGkZi_rxtmYqVsnEw9dKS9OmFvhLfGnFwDAAn6d5tZsJnRCrOseTR29IaRd7HVCvVL_lY5BDpl7XS8_u5vl6Us3dqvu-0bngH22Ngdead1QqMV58fFwXIkxKcMtMCgCNA0aqXt5Y_fhV7ClFTDxloby5GtxjLUhPalP9IOfrcB9q3M9fh65z5Peur6flRbMncD0TFT8A7Lphno6m-z9-qPgKYDgKN2Iw4BjbaefaaHR3B-sNTU5wLMusy=w1048-h1142-no?.png)
![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/wN_bfLTiL_1pZwl-q_gQqZUe8hP6uhYr-FAedDdzGF-K8bClsrPKszNlV28ZiSLinqOscV1iddnH7b2CKfzNCZkl1LsQ68_zAUbKlypPCZwzPHOlaxnzrL7e1cvvmN20KJ_wfvYeeA0jZSQY07iRhIy3tk8Rno-iPuJa0OZ9dDicQHFSczWXf2a-L0VXJgcjCXZTQxDuDRRvdA2HK8SXzbhwySzSSjhki-8zG0VlVQMzOhou9EMEkKf12OYYOVIq-2wf2cYCdsXaWCAPNLMiFOAGWlxKmdhrOD7VcXk51ZVPcFO_LaTFfTBIF890cMmZljpeMRtzIqZxi3Ndi1YHMq9_mT4WepTDg6gcIEzPeB4mibUZwtx3Ze45JdTEJItsYjbjaFgw1KaOnP_aKEdRv4nE0PsUjVq6hMl0ApG7rEqGUIXq-IPG2D-tslYGOywY9wVJPuscWO8HedPTDJ_MGAZ1lwDB0YeKG4Z-AfixqyrWeLufcQzXhOCd-CjRXfmahwdZKGLS9XVni0fjEAUAbEHp8cUXXdEBNCgCIadYjEkjXi7y7md_voqElUS6Dum9HNgf4YL7XJJqnhAmfGQTvEY8gh_RzZUTF-jLJS_aswkrpF87SqPsgIC1N450v3Qp=w734-h1715-no?.png)
![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/_0o3q-S0HkWdLqX51HI8ZQU0pQFudNVRhVkNGvgOuKp5skxB4LKA6Bzk9H5uYvP60wYJoSzPgADInjZTc1j4f824CPlu2oQiOXS9tlp6-h4PeKa7rl0b7fkEu1miSidsBALH3_FDqq-vL-yGGeryh5vG75e2d8btE5xsHjfbxoj8PYbvLlZOndgM1YS-Nm-NKH_J8wPPPsmI2Pc4npqvgekHSonxGrP0Y-75pNs90wdEb_NfICFZS2eOCn0xtMtNRKghmPSHFfhNJPpNIxch5-Mj-lqDKELhlY7kyNQ8bpAjG3tdFWGaeI71kfpJj9y94Xb-LKUm5oEQmGcTiRKUczG13Xq9oAoEEx0wboMD-BtfeiRlGXn-FhHuQ_TRfS1YIWpheeyLlH7cKyvbEJR_m8aYabq-1OHRAt88-91NZiSc7h0BS8iQ1NzhgZOwY9OV40CsoZWT8QH6EGMSDQe2BgujAtStBUvS8jbxxM7ffj-aD5kHJzj4fkd-e2agYbJnHxTJFOEgUhjW0WxbFiIddEXjInJ1-pUuw9BE1jeUCe4zaKW-gduAYS7RKmqpo4PjUsNw6TVUHiQUEoVB4Czlo-sjlLYHr6ePbuIQsdfHIDZ8VrWILQGBxVZLe1xT79Yy=w1185-h1087-no?.png)
![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/Q7boz4eB73qjaEiI4r3pUCQn3gZrdqAfye7YAS4Vo_9DnAuOgEgPMuyIQAdHrYEGfOrIjZF3KmHvClCMmMzpbq-4hGVpcQA72q4wSYxPx5qH62dHaSeRIvFlrda1lkcmbvU6Y9bN_gWdtl9eWd0UueWTYI_QnyZ2_gdlcsElnAMFGwklnTfYKOhWqEQOTOliN8wzYJlDoXcxp3rJijE7i0dNNFEfoOuAvvuOqRo7CiaI_JvWNpgzUJdAIZzrGHax4GLZ9R07LqwL10QFUmjfqHl7HRhkuIHx5rzIsyKTZKDaeK_0D5oC0kbb2aEJsjEL_4w6xmhKtCUw7KL2fvIrj8Q083rXg7-NtfoGj1yv1z9uyIyFPAQbcU-zdJGqrC-QQt3rpHfLyphsvOM8TBhIB2xzMtc4bXGgFXclxyX14CvRRg0w27_y7xHuu594OLS1TjMqWYrkY3nm3lllYbF4Z5ngWySmn4M3wAxJK2qR58vI5ZBWzjV3GxSSFrZofwEhW_2S3QkkbMXHsBe1R7qnDbSLkhUHvc2cn7WgnDCedlmFIis3Pq4KJkbjqVNGcSF66sL3JXhA78usR4MJcd4nmtmiZsckRdHQe9Sazg_Pi7BWGLpOWSq6EwqrHHrSTlGe=w1001-h934-no?.png)
![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/FRlCOYrbwUvCScQhFiLAH73TGNwP0AQ2kpictoEK_2H0EULO-4h7oY6BZ8X6wg9PI47etYP03ilDW_ZpnS5L-QSq7PU0l3Juou_HNhvCj0iUOguyxKHCqP7py50emRSPkwqySuhyL_FyMql1kz9F5cxSIA-3nWQpa4gtY9RECi4MTmZ1Y3AQkpsTkXdBpbK5nuSR44uihC-EYXUPcyUd8CWx53zRjQBZf7gTdJ7MRVdk18iXcyjMgXRN5nLzznKOgm8NCuqhb7UqSrrM81PszlyETSuIwX1sHFm35vIPiY6Ldomot6wzsOhDTnXewidEHo-w_4raMuNKnvT2m_Ij3oaFeAY0CjxxZWoNCGexhO7J6eJ7wGSz8KrpPIXi8cpSRSIn4qzKd08qfd9dyurjlEZuTU1Ildto2PC2sYmR09huQ5vB18T7XngOajsnmqLjqwqAtilokNIs4KgF7mivko089D8mEYVFaGHzvJjAwVqw1NCgXAmx77t6h6PrJIyq5EhLAuJvVGpc01TcY4sRmlWkYF_VJuL14hkaDx6tpKbi23vvTXN1bmRf7njNoxkP-L86iAPccuZ5zSvmfYrGhbVdcmrt1UEhkYcV4Xi3wzggQBSTEXmNIQ5Fq03ipxyM=w708-h532-no?.png)
![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/XFDl0q0Rwt0ieE62MC3QTB8VYP0mu1-uY798DcmQKnd_qqxPnW7nQLkAgUmJ6cPsr777-T6hPyPsCtxJkUctwYSq3g2MT8zHzyIsZdHeHNCcIOYAw-_n7lenWJeCGq80HezlJq3OgMwLgOipEYVJKfzK0zbq1d3tRInfxvLs3zU88ttGO2j8ysEfPEXPTG71hatRz0Rstl3leFxfgXzIyxOJXgZp2SIIsPUwx7_Ecq_GttnguZma6WpvDRTs15YDSPGaZADVRrb9BFfTfebfmpGEHqka9zv898qiSZxaSMB5PJmMEhgFebulIgnbuyqhUqmH42KzikLgS0z3Zh_0CIbRv5RlnTCQSX9JDxPL49EO_zUrWQ7-SNGa2ttgZX2kZJuC4iYlqiDft-SABsSKJhzSg51JztCIsOULWjGfAV0_1CiisIz0i3WwZTK1gGzSxbyyCtrTzHifU6-OpDsp8wSWS3cZgOOFB5nnw6CuYB3Z1_QZN7utQPA_GMS5pKKdvp0OJzq3eGv2jmkP80ee8XFesTYX6bzU2b09hVCnehgb4_GyiSf7a6QNZnqQFvskR21452Y7xcOiQzXHUOmLv45njCUED3S9nATb-lkz6htvMBTAViPkIsId8yaXh9hM=w1442-h1804-no?.png)
![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/KGGYPWHcCbAK4oeSGofzamLQLBpyvwtdwUbpXs5moOrhnDkdxf9AySPDnNnrNltbQyBzO16pPlxPwHlWFz-UNkRPtbsY0SFOe9CsxrasJLG9ZZo7u9pg-WRvh4lgQ1AtIvF8-Tgk_5V__8swNJLsZYGprx85uXFFsfg9BS_BmW2XBEez-YIfPq9uX1J_K8LfyCnRsTWd7JCtn0a5drNMMw72_0h0yDTXMVEHjwHhoY5zbAF1M-c-yrZmPH5aToXLt4ay3NkpVKQKxxIMIcHvTS55LxSnuKO4UTQWBsDDX_gZZR7FpVwlWXa7PRqFQzVsWN5CTrzOOi1Qp_tSCuTqCGkJy6SzKVJ-e43ztGKaO4QHTOZ2XI8rpEpl9ljiyCvDZH7EuYynB6CK1TEmnVUH64my4GTNWWEXr5Hi8YpUSZqdCRvBB4Wt7zODKBzyzKs_8hmBRRGwo7dXDFCintt-PjMNbmDmskI4_t0itQgjZx8l1x2Q51hcnw8CO24VcVR9j_9d56AYo1i4VpdBUdwIs4V3v2TxGXnlu6Biel8n-J0aJUsnxzDKZf7vwDAgaOr6Th742DFYbPjMcgaP715r_d5NOwEWwi5eDkdvbnriGRpgEDBPm6I-pOwjZS0DUWxb=w1433-h470-no?.png)
![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/gtYtIUMOOj63s49qyU-Rmvi50X5G5MS0pmxQ-y-ELZ8P4BRmNa6dQbuqzmbCZaww5gl4pQqbe1D0a4RBDbeU8V69VoSAvXtXh9lSA-7Ndyj5rmLHzm-zXRj33nAOCqv2ekCBKWGfcphjCIeWw9H45oBwAHP9EP4f7nUS0dAGjX8YAYjlBlu2sZ6GMv_WCotzl3qpx03OLI_o_D0E_L3wyKYQqIvjxKzPZRfZqzx5qC7Fpe6dW0ITt49ZNyo_41lGzP61MWXzVLP-b3eQiNoQDeroyj72FCFNi90NW45i6JLIsjMjT6qfFQx9tuw14_4gLMAgMw7ouwWnlM5Cly1h8qpsTzErn2STt9O1DE7N7gIAOIC_rh5vi-dbp64xZegUkrf1SH2tctip-bVBJB6-dX6NPPQCxc6gybpC7D9Gd7EVjFOLmi3gBfKNlHGgS6bst0LWXQ02_mNkGYQzBYdx6KFlq66KUI_D8eedji_Sg44gRlDwNES4j2dNPnu62oZKUiKbZCVaHnI5ubBVqfLCUR_OJ8LpfxMyJrytI_wqCQV_Ce4pX92d91tlRO1ub6otIEJ7LHmxZuPWHxvdL8phn0X5QAN_ujBGv8zmb39J58AIebuAJx3YeB4KOpYalXB7=w702-h1128-no?.png)
![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/LGvJ9I_ijwWSV8n92Wr0QShT5aZfwl5_a-e4928VS1BKgi7ixkMXTWNdhoR7-GuRzOBiDAfb1bEXZlCw4_xGJqCJ2wEMJw58JPrG5lExKFO-art6sSfeyr_3DYfuAKyTh9IItj1c0Q6UZ1t7c7I3tFcpBRLBfNHG7lLXd_8kVnk60C6HvsFeNvuc9jXC0FoAN9fXnVVNOwu9Q9pPfyibxxbX_VL36pY8viLbeFeECvaTwF8mrksDeHVOcKsAkpLLGR-vgUmwGQQwoyorOTOwJHuPNBmxgu1cmIVxPd7NPCyU5UcSFhqWWXSje__Cb14460OvyLI7YRRY9K_3qmZBqJLTYLD6jNooQbfqF00jn8YSqamdBjJvjdhfDTUYm4wPntAmP1oeIKk3lfh7_3aFzqj3iIs3b4jOJAjjPiZuDDN1XgxmIZm9LVNUeVBmPa9LxJeBaG8P7cCOO8l6El2fuRmxfrOGgjFiHjlVMKIhUy6EsNMhf3ucHx7dBVz9TX32snA1nV_pSNer0-vwotqBY6gv9KIPjrA2joGBSs9doJdv_Ha9gu7Iu5Fk7jTQanQ5d2p_KIrD29CQufMBow6LTmfnVkJyiG8Tw8Hm2vb14THPGTSjgdsIOOf11w-1SaYs=w699-h460-no?.png)

![[Linked Image] [Linked Image]](https://lh3.googleusercontent.com/qgB3scqd0ToCneBecFUbwN6u6cH9tGWV8YOnoRfp7zdlbr8bhC5l0Kh7ke0gMTlnDvdR_9_LCotrdV5ha9-p_7n-BnGCx4kj12PM0moxBS3dZLZgwDLhfzt-7dIUR5lXXjBNU6kBlNUCjC80LYnm_ESVALq5gXvjuWDoevGvwBpACyA1MMXVT1CUqGU8Lf8AkeC6q4arCPzvE7-uxfjeJasFcAopiWeKy8cTOpFP9749cjtJruq6OcnzauesIOa2KxYuaX3fAmGGbZVdpmpcVpZKMfyJVJHLD7yK6YuV347wnIqkngiT0ddP2nmDYJTxDZC9huMa9uIwlwg0bEMudN6eSuq1SUpDexW8wf5dkl7A5pU2vp8CVTwdEEGH5rtYXP0CFcGHc0ve4tOAeFxJP8L3Qa_hgzXjzuUj-YcRz7pZa7DP7ZbdS6euQj4Rr042mBEKglp8A6hSXOiLDlbN7ExE4g-Xu7J2Xcx3OKpIpey13UtRPJv06PSbP5VqCNgMP8RZTrk3O5rYjdN0NkYX5N0OY27ZvDmXBEu8PTsvSjTjIiC72NOj8hZ1YUbSVE-EW010Rv3rkFq_uuICuOXd0GdYUCZTHPaWbM0EFISQ4Sz6AwgnSwwlHAR6Da5bq2rz=w433-h278-no?.jpg)