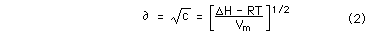Well, thoughts of mine then

When it comes to lubricants viscosity, there are several important aspects of engines to consider. As mentioned before here, one of the main challenges lies in refining surface roughness and reducing bearings clearance.
Over time, clearances have generally decreased, with German engines holding no particular disadvantage over American or Japanese motors in this regard (Why they need more viscosity?? Please abstain from expounding upon temperature, for the question served but a rhetorical purpose.)
Now, let's explore the significance of viscosity reduction and its impact on engine oils.
By using oils with lower viscosity, we can achieve a few benefits. Firstly, the oil flows down more easily, which is a small but positive advantage. Secondly, and more importantly, lower viscosity oils have greater solvent capacity.
In modern engines, issues like oil coking and piston ring sticking have become common. These problems can be attributed to various factors, including the quality and viscosity of the oil. While the amount of polymer & additives in the oil plays a role, it's not the most critical aspect. It's possible to produce SAE40 oil with a small amount of polymer, but for certain applications like PCMO in winter, mineral 600 base oil without polymer is not suitable.
So, Law: when we decrease viscosity, we enhance the solvent capacity of the oil due to the physico-chemical properties of liquids. I am comming to realize that there is no need to embark upon a daunting search for identical oil series, meticulously comparing their D611 and D1133 characteristics, It suffices to say that reducing viscosity leads to increased dissolving capabilities. But I guided by the profound wisdom encapsulated within two fundamental equations: [Stokes - Einstein] and [Nernst - Brunner]. Should you desire, I am more than willing to share the some insights of these revered equations.
Boosters like esters can be used, but they also tend to provide better results in SAE16 oils compared to SAE40 oils. However, it's important to note that even boosters have limitations. Esters have limitations in PCMOs a lot.
In essence, within a chosen series of oils with similar compositions, lower viscosity oils always exhibit superior dissolving capabilities.
An "average" SAE16 oil can have solvent capacities up to 1.5 times better than a chemically similar "average" SAE40 oil. Moreover, the starting temperature for dissolving forces and optimal dispersibility is much lower for SAE16 oil, often at around 85°C, while SAE40 oil requires temperatures of 115°C or higher.
In conclusion, reducing viscosity can significantly improve engine cleanliness and the performance of piston rings - both crucial factors in modern internal combustion engines.
Now, we must come to terms with a singular truth: contemporary low-viscosity oils are crafted from remarkably tenacious and non-volatile synthetic bases, rendering them impervious to the volatility that plagued thin oils of yesteryears.