Great thread everyone, thanks.
Just to add to it, here is an interesting article about ZDDP by Penrite, giving an interesting historical angle.
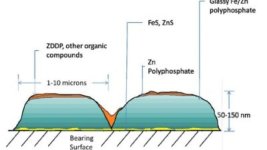
ZDDP (Dialkyl Dithiophosphate), is a chemical compound used in engine oil as a sacrificial and very effective anti-wear agent. There has been a big focus on zinc, also known as ZDDP or ZDTP (zinc dithiophosphate). For many years this has been the anti-wear additive of choice for many engine oils...

penriteoil.com.au
"ZDDP was developed in the 1940’s initially as a bearing corrosion inhibitor before being used as a sacrificial anti wear agent for engine oils. From the early 1920’s to the mid 1980’s petrol contained tetraethyl lead which contributed to the build up of lead & lead oxides in the engine. Lead scavengers were then introduced but these caused acidic by products in the crankcase that reduced the effectiveness of the ZDDP. To counteract this, higher ZDDP dosages were introduced often into the range of 0.14 - 0.16%. The increase also pushed up levels of Phosphorus which is part of the ZDDP compound along with zinc.
......
There are many and varying opinions on what levels of Zinc are needed to be an effective anti-wearing agent in engine oils. Owners of vehicles that have flat tappet camshafts, veteran & vintage owners and traditionalists may argue that the higher the level the better especially in vehicles that do not have catalysts. This is not always the case. As we saw above, ZDDP was increased in oils to combat the effects of lead scavengers not actually to increase the anti-wear protection.
In effect, an engine oil that contains about 1000ppm or 0.1% phosphorus (approx.1100-1200 or 0.11-0.12% PPM Zinc) or higher, will easily provide the required anti wear properties for older engines. General Motors experimented in the mid 1950’s with lower phosphorus and zinc levels and found that 800PPM or 0.08% percent phosphorus level (approx. 1000 PPM or 0.1% Zinc) eliminated many wear issues. In fact, they also experimented with oils containing 600PPM or 0.6% phosphorus on mixed fleets in the 1970’s and found no wear problems."