Originally Posted By: Bror Jace
Like
Jax hinted at, I think the additive package in Red Line Oil is a bit too aggressive which offsets the protection the esters and all that moly might provide.
I choose other brands for daily drivers.
The "aggressiveness" you refer to of this oil has often been cited by the tribologists and other knowledgeable people such as Terry as redline's cleaning of oxidized metals. It is not necessarily an indicator of actual wear. This has also been cited as the reason why the 2nd or 3rd redline sample shows diminished wear of some of these metals.
Also, it seems to me that if the benefits of moly (some sources such as this
http://www.bobistheoilguy.com/forums/ubbthreads.php?ubb=showflat&Number=1505101#Post1505101 show 20-40% reduction in friction and wear) are to be realized, then the oxidized metals, etc. need to be cleaned off so that the moly particles can fill the asperities. So the "aggressive cleaning" seems to be part of the formula that redline is using to reduce wear.
If anecdotal evidence is any indication, it does seem to work. F.e. a saab turbo that had redline it's whole life when I sold it to my friend at 318k he was going to rebuild it because he believed no engine could go that long and not need a rebuild. At 318k it looked new inside and everything was still within factory spec.
Also here is an anectdote from BITOG member Barkerman:
"Red Line is a good daily driver oil and it just might make your engine last longer. We have several 300k+ cars around here that use nothing but Red Line and it's not that they are over 300k but that they are over 300k with no internal mechanical work and to a boroscope look like new inside. I know a few examples don't prove a point but they are a good indicator. One fellow delivers some kind of radio-active isotopes with his 78 Toyota pickup and has 510k miles on Red Line with nothing more than regular maintenance and about 4 water pumps. He also uses Frantz oil and fuel filters and a spin-on coolant filter with an anode. He is on the original camshaft and valves. I've done a compression check and it's withing 5% of new specs and the spread from high to low is 8psi. You can still see the hone marks at the bottom of the cylinders and there is only a cosmetic ridge at the top of the cylinders. Granted this series of Toyota 4 bangers is considered to be a good engine I think that Red Line is performing well. He started with Castrol 10w-30 with the new truck and a few years later after hanging around our shop switched to Red Line 10-30 and has graduated up to 20w-50 a couple of years ago. His oil consumption is 1 quart in 5k miles. Granted he is a careful driver because of his cargo and LA has no weather but I think he is doing well. He recently dropped a bundle in rebuilding the suspension front and back, complete brake job and replaced all the flexible brake lines, master cylinder, rear wheel cylinders and new calipers, including his second tranny rebuild for bearings and seals, no other hard parts also using Red Line MTL. ...But to those that think Red Line is only good for racing we think it performs well for commuting but is an expensive choice. Sorry for running on I just though this is an unusual case and you might be interested."
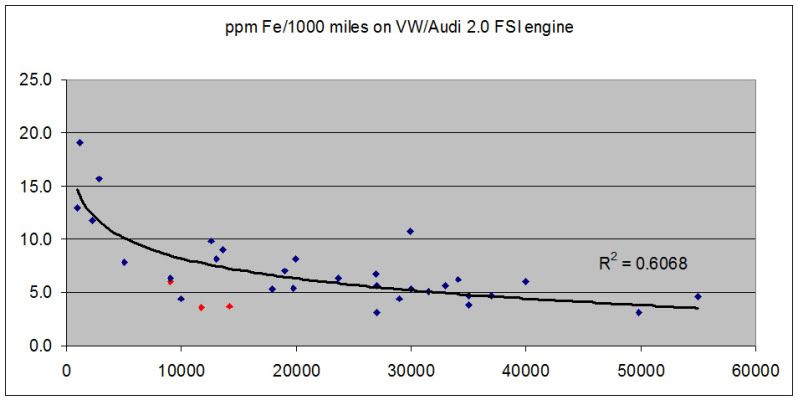
Also, redline (shown in red in the graph below) has shown low wear numbers for fe compared to other oils on the vw/audi 2.0 FSI. But this is really limited data as there is only one car running redline (mine) but it is driven hard and the first run was 90% 1-2 mile trips in the winter.