2001 Saab 9-3 SE
Mods: 3inch exhaust,Stock boost (15psi)
Temps: Dec-April -30c to +15c (mostly -10c ish)
Car has an oil sump heater which was used everyday(heats the oil to +40c), almost 50% of cold starts had the oil heater on.
Mileage L/100km: 8.0-9.0L/100Km
Kilometers on engine 146,000
Kilometers on oil 8,000
Make Up Oil 0
Amsoil Ea026 filter (added 2 Liters to a total of 6L )OEM is 4 Liters
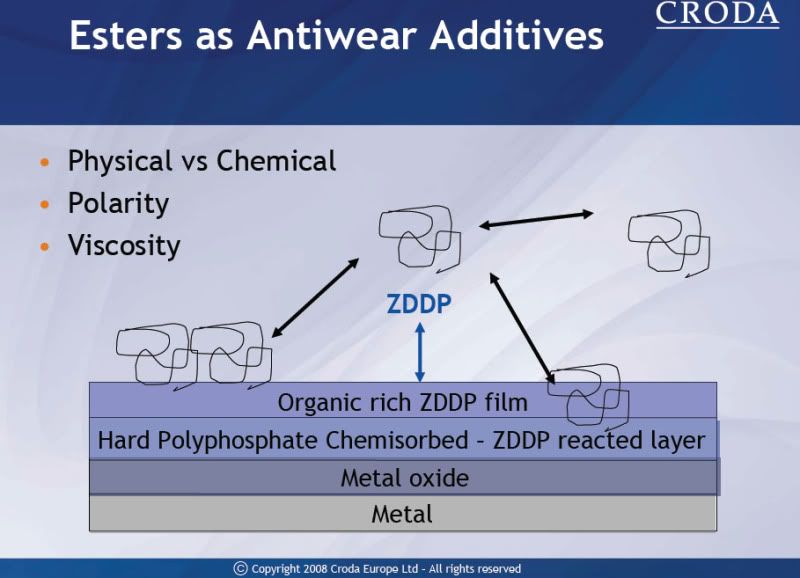
I really liked this oil, car seemed to run smooth even when it was really cold out, will be sticking with this oil for next winter. PS. I love the oil pan heater, car starts as if it was summer temps.
Redline 0w40, 2nd column Motul 5w40 8100 xcess
Aluminum 2/2
Chromium 0/1
Iron 10/10
Copper 4/6
Lead 2/2
Tin 4/1
Moly 522/4
Nickel 0
Manganese 0
Silver 0
Ti 0
Potassium 2
Boron 58/2
Silicon 27/17
Sodium 17/12
Calcium 2015/1553
Magnesium 72/771
Phosphorus 895/837
Zinc 1055/1069
Barium 0
Sus Visc @ 210F 72.7 should be 55-62
cST Visc @ 100 C 13.65 should be 8.8-11.1
Flashpoint in F 400 should be >365
Fuel % Antifreeze 0.0
Water % 0.0 should be Insolubles % 0.0 should be TBN 3.7
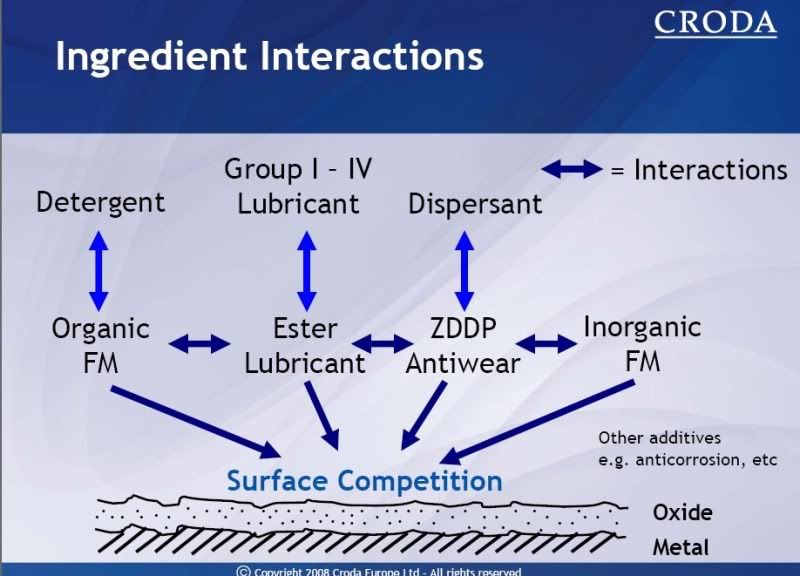
Black Stone: This Red Line 0W/40 contains quite a bit more molybdenum (anti-wear additive)
than the last oil that you sampled. After this most recent oil change interval of 8,000 km, wear continues to
look good here and no harmful contaminants were found. The viscosity of the oil read in the 40W range.
The TBN read 3.7 so you still had quite a bit of active additive left, though not as much as last time. We
think the silicon here is harmless silicone product, as wear wasn't affected by it. As of 07/01/09, we have no problems to report.
Mods: 3inch exhaust,Stock boost (15psi)
Temps: Dec-April -30c to +15c (mostly -10c ish)
Car has an oil sump heater which was used everyday(heats the oil to +40c), almost 50% of cold starts had the oil heater on.
Mileage L/100km: 8.0-9.0L/100Km
Kilometers on engine 146,000
Kilometers on oil 8,000
Make Up Oil 0
Amsoil Ea026 filter (added 2 Liters to a total of 6L )OEM is 4 Liters
I really liked this oil, car seemed to run smooth even when it was really cold out, will be sticking with this oil for next winter. PS. I love the oil pan heater, car starts as if it was summer temps.
Redline 0w40, 2nd column Motul 5w40 8100 xcess
Aluminum 2/2
Chromium 0/1
Iron 10/10
Copper 4/6
Lead 2/2
Tin 4/1
Moly 522/4
Nickel 0
Manganese 0
Silver 0
Ti 0
Potassium 2
Boron 58/2
Silicon 27/17
Sodium 17/12
Calcium 2015/1553
Magnesium 72/771
Phosphorus 895/837
Zinc 1055/1069
Barium 0
Sus Visc @ 210F 72.7 should be 55-62
cST Visc @ 100 C 13.65 should be 8.8-11.1
Flashpoint in F 400 should be >365
Fuel % Antifreeze 0.0
Water % 0.0 should be Insolubles % 0.0 should be TBN 3.7
Black Stone: This Red Line 0W/40 contains quite a bit more molybdenum (anti-wear additive)
than the last oil that you sampled. After this most recent oil change interval of 8,000 km, wear continues to
look good here and no harmful contaminants were found. The viscosity of the oil read in the 40W range.
The TBN read 3.7 so you still had quite a bit of active additive left, though not as much as last time. We
think the silicon here is harmless silicone product, as wear wasn't affected by it. As of 07/01/09, we have no problems to report.